Page 10 - Curriculum Visions Dynamic Book
P. 10
Changing one element into another
The change from one element to another is called transmutation. Remember that atoms are made up of different combinations of protons, neutrons and electrons, so the break up of a neutron leaves behind an atom with a different combination of neutrons, protons and electrons.
This new combination is a new element. So when uranium loses nuclear particles, a whole chain of events is set in motion until finally lead is created. Different forms of radiation are produced during
this process.
The chain of events tends to happen very slowly, sometimes only over many millions of years. As we shall see, this slow rate of change gives rise to one of the main problems of dealing with radioactive materials.
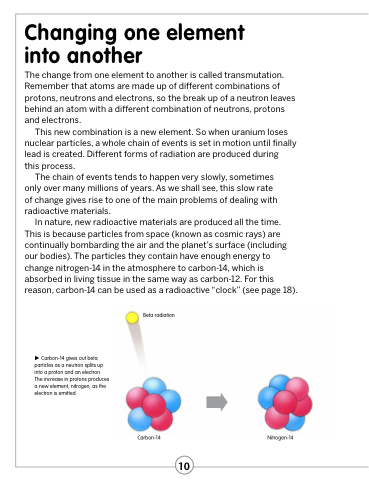
In nature, new radioactive materials are produced all the time. This is because particles from space (known as cosmic rays) are continually bombarding the air and the planet’s surface (including our bodies). The particles they contain have enough energy to change nitrogen-14 in the atmosphere to carbon-14, which is absorbed in living tissue in the same way as carbon-12. For this reason, carbon-14 can be used as a radioactive “clock” (see page 18).
Carbon-14 gives out beta particles as a neutron splits up into a proton and an electron. The increase in protons produces a new element, nitrogen, as the electron is emitted.
➡
Beta radiation
Carbon-14
Nitrogen-14
10
10