Page 8 - Curriculum Visions Dynamic Book
P. 8
Types of radioactivity
Proton Neutron
There are a number of ways in which a nucleus can changes. Each change shown here produces a different type of radiation.
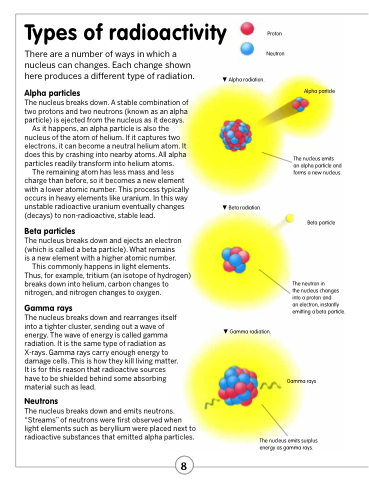
Alpha particles
The nucleus breaks down. A stable combination of two protons and two neutrons (known as an alpha particle) is ejected from the nucleus as it decays.
As it happens, an alpha particle is also the nucleus of the atom of helium. If it captures two electrons, it can become a neutral helium atom. It does this by crashing into nearby atoms. All alpha particles readily transform into helium atoms.
The remaining atom has less mass and less charge than before, so it becomes a new element with a lower atomic number. This process typically occurs in heavy elements like uranium. In this way unstable radioactive uranium eventually changes (decays) to non-radioactive, stable lead.
Beta particles
The nucleus breaks down and ejects an electron (which is called a beta particle). What remains is a new element with a higher atomic number.
This commonly happens in light elements.
Thus, for example, tritium (an isotope of hydrogen) breaks down into helium, carbon changes to nitrogen, and nitrogen changes to oxygen.
Gamma rays
The nucleus breaks down and rearranges itself into a tighter cluster, sending out a wave of energy. The wave of energy is called gamma radiation. It is the same type of radiation as X-rays. Gamma rays carry enough energy to damage cells. This is how they kill living matter. It is for this reason that radioactive sources have to be shielded behind some absorbing material such as lead.
Neutrons
The nucleus breaks down and emits neutrons. “Streams” of neutrons were first observed when light elements such as beryllium were placed next to radioactive substances that emitted alpha particles.
Alpha radiation.
Beta radiation.
Alpha particle
The nucleus emits
an alpha particle and forms a new nucleus.
Beta particle
The neutron in
the nucleus changes into a proton and
an electron, instantly emitting a beta particle.
Gamma rays
8
8
Gamma radiation.
The nucleus emits surplus energy as gamma rays.