Page 11 - Curriculum Visions Dynamic Book
P. 11
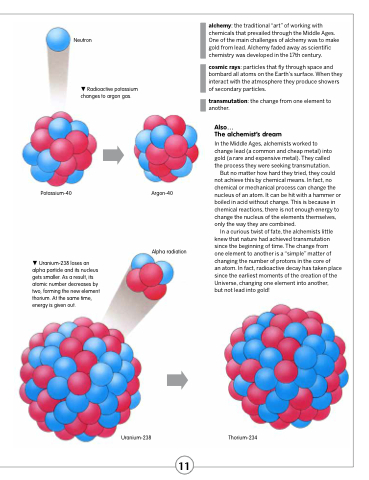
Potassium-40
Uranium-238 loses an alpha particle and its nucleus gets smaller. As a result, its atomic number decreases by two, forming the new element thorium. At the same time, energy is given out.
Argon-40
Also...
The alchemist’s dream
In the Middle Ages, alchemists worked to change lead (a common and cheap metal) into gold (a rare and expensive metal). They called the process they were seeking transmutation.
But no matter how hard they tried, they could not achieve this by chemical means. In fact, no chemical or mechanical process can change the nucleus of an atom. It can be hit with a hammer or boiled in acid without change. This is because in chemical reactions, there is not enough energy to change the nucleus of the elements themselves, only the way they are combined.
In a curious twist of fate, the alchemists little knew that nature had achieved transmutation since the beginning of time. The change from
one element to another is a “simple” matter of changing the number of protons in the core of
an atom. In fact, radioactive decay has taken place since the earliest moments of the creation of the Universe, changing one element into another,
but not lead into gold!
Neutron
Radioactive potassium changes to argon gas.
➡
alchemy: the traditional “art” of working with chemicals that prevailed through the Middle Ages. One of the main challenges of alchemy was to make gold from lead. Alchemy faded away as scientific chemistry was developed in the 17th century.
cosmic rays: particles that fly through space and bombard all atoms on the Earth’s surface. When they interact with the atmosphere they produce showers of secondary particles.
transmutation: the change from one element to another.
Uranium-238
Thorium-234
Alpha radiation
➡
11
11