Page 15 - Curriculum Visions Dynamic Book
P. 15
Also...
The zinc dry cell is one of a number of primary dry batteries that use zinc.
In addition to the inexpensive zinc cell, alkaline, mercury and silver-containing zinc batteries are also made.
The only difference between the alkaline cell and the zinc cell is that potassium hydroxide is used as the electrolyte. With this modification, the power output can be kept high for a larger part of the cell’s life than with an ordinary ammonium chloride electrolyte.
Mercury dry cells are not as common nowadays because of concern over mercury poisoning. Mercury cells are essentially alkaline cells that use mercury oxide as the central terminal. Like other alkaline cells, mercury cells will maintain a steady voltage over more of their working life than ordinary cells.
Cells made with silver chloride or silver oxide are also popular for special applications such as hearing aid batteries. Silver chloride is used as the positive terminal and zinc as the negative terminal, with zinc, sodium or ammonium chloride as an electrolyte.
electrode: a conductor that forms one terminal of a cell (battery).
electrolyte: a solution that conducts electricity.
oxidation: a reaction in which the oxidising agent removes electrons. (Note that oxidising agents do not have to contain oxygen.)
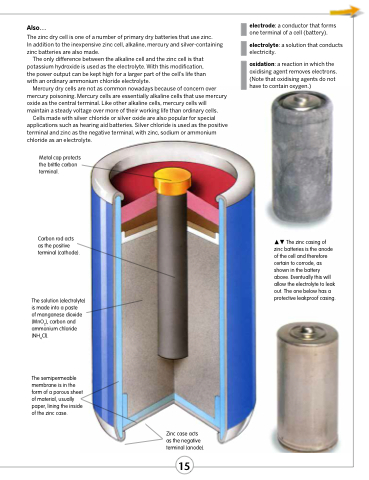
Metal cap protects the brittle carbon terminal.
Carbon rod acts as the positive terminal (cathode).
The solution (electrolyte) is made into a paste
of manganese dioxide (MnO2), carbon and ammonium chloride (NH4Cl).
The semipermeable membrane is in the form of a porous sheet of material, usually paper, lining the inside of the zinc case.
The zinc casing of zinc batteries is the anode of the cell and therefore certain to corrode, as shown in the battery above. Eventually this will allow the electrolyte to leak out. The one below has a protective leakproof casing.
Zinc case acts as the negative terminal (anode).
15