Page 13 - Curriculum Visions Dynamic Book
P. 13
Secondary batteries made with zinc
A secondary battery can be charged from an external source, causing a chemical change of the surface of the electrodes. When the battery is connected to a load, the chemical change reverses and electricity is given out.
The most commonly known secondary battery, used in cars, is the lead-acid battery. However, zinc–silver batteries are also used as secondary batteries. They are much more expensive than lead-acid batteries,
but will produce a high current very quickly and store about three times as much electrical energy per gram of battery compared with lead-acid batteries. Their main use is for military purposes.
Cadmium–silver batteries are used in the same
way as zinc–silver batteries. Neither is able to store a charge for a long time, and chemical reaction occurs on the plates, resulting in a loss of charge. Zinc–cadmium batteries discharge at a high rate on demand, but they are also able to store a charge for long periods.
The electrolyte used in these secondary batteries
is potassium hydroxide. Each zinc–silver cell produces 1.6 or 1.8 volts (depending on the oxide of silver used). Dry zinc–silver batteries (where the electrolyte is made into a paste) are sometimes used in hearing aids.
anode: the negative terminal of a battery or the positive electrode of an electrolysis cell.
cathode: the positive terminal of a battery
or the negative electrode of an electrolysis cell.
cell: a vessel containing two electrodes and an electrolyte that can act as an electrical conductor.
electrode: a conductor that forms one terminal of a cell.
electrolyte: a solution that conducts electricity.
electron: a tiny, negatively charged particle
that is part of an atom. The flow of electrons through a solid material such as a wire produces an electric current.
ion: an atom, or group of atoms, that has gained or lost one or more electrons and so developed an electrical charge. Ions behave differently from electrically neutral atoms and molecules. They can move in an electric field, and they can also bind strongly to solvent molecules such as water. Positively charged
ions are called cations; negatively charged ions are called anions. Ions carry electrical current through solutions.
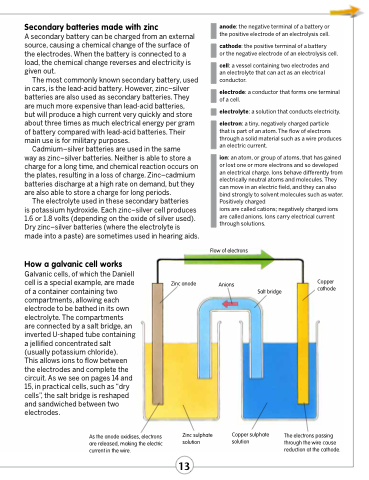
How a galvanic cell works
Galvanic cells, of which the Daniell cell is a special example, are made of a container containing two compartments, allowing each electrode to be bathed in its own electrolyte. The compartments are connected by a salt bridge, an inverted U-shaped tube containing a jellified concentrated salt (usually potassium chloride).
This allows ions to flow between the electrodes and complete the circuit. As we see on pages 14 and 15, in practical cells, such as “dry cells”, the salt bridge is reshaped and sandwiched between two electrodes.
As the anode oxidises, electrons are released, making the electric current in the wire.
Zinc anode
Flow of electrons
Anions
Copper cathode
Salt bridge
Zinc sulphate solution
Copper sulphate The electrons passing solution through the wire cause
reduction at the cathode.
13
13