Page 17 - Curriculum Visions Dynamic Book
P. 17
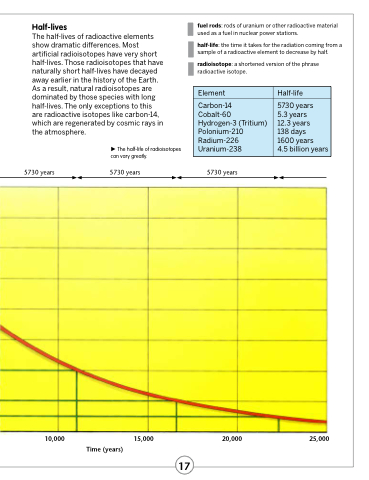
Element
Half-life
Carbon-14
Cobalt-60 Hydrogen-3 (Tritium) Polonium-210 Radium-226 Uranium-238
5730 years
5.3 years
12.3 years
138 days
1600 years
4.5 billion years
Half-lives
The half-lives of radioactive elements show dramatic differences. Most artificial radioisotopes have very short half-lives. Those radioisotopes that have naturally short half-lives have decayed away earlier in the history of the Earth. As a result, natural radioisotopes are dominated by those species with long half-lives. The only exceptions to this are radioactive isotopes like carbon-14, which are regenerated by cosmic rays in the atmosphere.
The half-life of radioisotopes can vary greatly.
5730 years 5730 years
fuel rods: rods of uranium or other radioactive material used as a fuel in nuclear power stations.
half-life: the time it takes for the radiation coming from a sample of a radioactive element to decrease by half.
radioisotope: a shortened version of the phrase radioactive isotope.
5730 years
10,000
15,000
20,000 25,000
Time (years)
17
17