Page 16 - Curriculum Visions Dynamic Book
P. 16
Half-life
Radioactive materials are widely scattered
in all the rocks of the Earth. Pick up any volcanic rock from the ground and it is likely to contain atoms of radioactive elements.
Radioactive elements send out radiation
all the time, although gradually the level of radiation decreases. However, whatever the level of radiation, all radioactive materials lose half of their remaining surplus energy at a fixed rate. Thus, the time taken for a piece of an element to lose half of its remaining energy is always the same, whether the radioactivity is strong or weak.
The rate of change is described by the term half-life, meaning the time it takes
for the radiation of an element to decrease
by half.
Decay: for ever
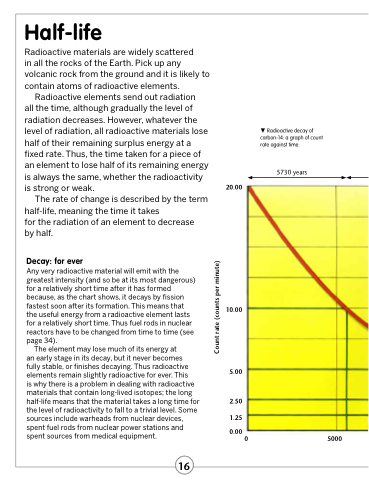
Any very radioactive material will emit with the greatest intensity (and so be at its most dangerous) for a relatively short time after it has formed because, as the chart shows, it decays by fission fastest soon after its formation. This means that the useful energy from a radioactive element lasts for a relatively short time. Thus fuel rods in nuclear reactors have to be changed from time to time (see page 34).
Radioactive decay of carbon-14: a graph of count rate against time.
5730 years
The element may lose much of its energy at
an early stage in its decay, but it never becomes
fully stable, or finishes decaying. Thus radioactive
elements remain slightly radioactive for ever. This
is why there is a problem in dealing with radioactive
materials that contain long-lived isotopes; the long
half-life means that the material takes a long time for 2.50 the level of radioactivity to fall to a trivial level. Some
sources include warheads from nuclear devices,
spent fuel rods from nuclear power stations and
spent sources from medical equipment.
20.00
10.00
5.00
1.25 0.00
0
5000
16
16
Count rate (counts per minute)