A group of atoms of exactly the same kind makes up an element.
Elements can combine their atoms to make new substances.
The word atom means something that cannot be divided into anything smaller. This means that an atom is the smallest particle of matter there is. If huge numbers of atoms of exactly the same kind come together, they make a piece of the material we can see, and we call it an element. Every solid, liquid, gas, and plasma is composed of atoms. Atoms are very small - a ten-billionth of a metre across.
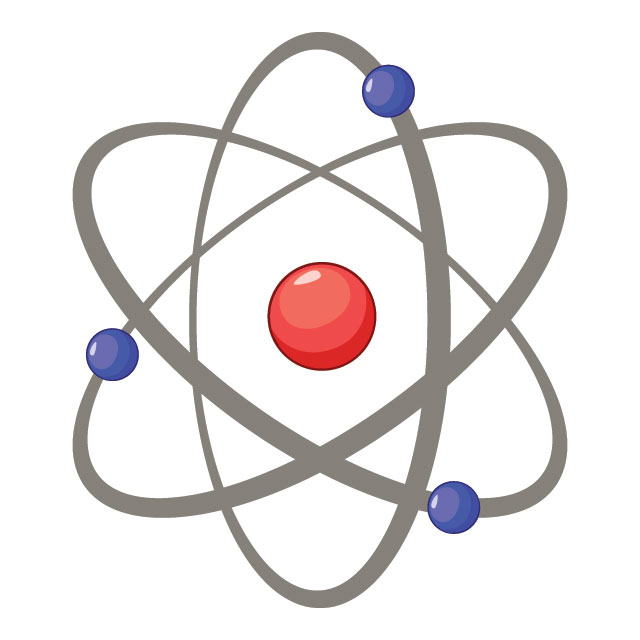
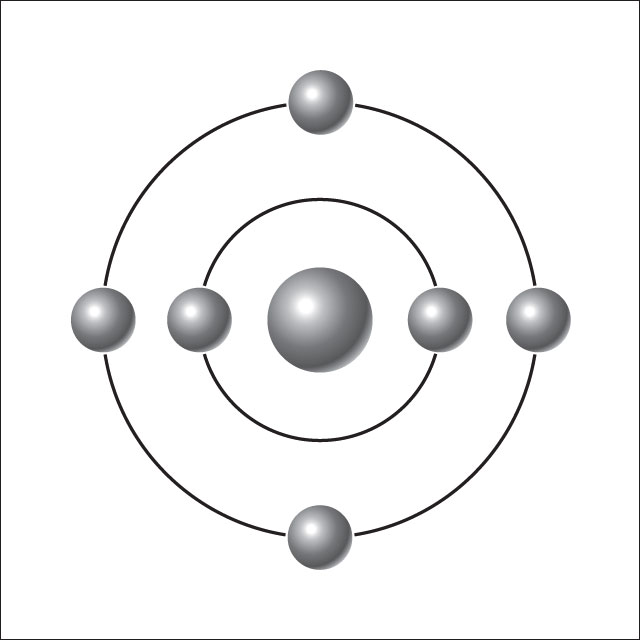
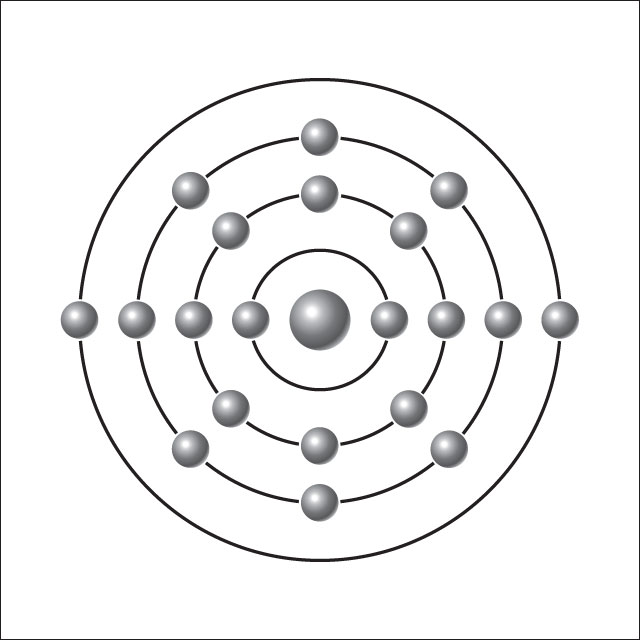
Think of an atom as being like a very tiny Solar System. The star is called the nucleus, and the planets are called electrons. Every atom is made of a nucleus and one or more electrons bound to it. The nucleus itself is made of two even tinier particles called protons and neutrons. More than 99.9% of an atom's mass is in the nucleus. The protons in the nucleus have a positive electric charge, while the electrons orbiting it have a negative electric charge, so the two hold together.
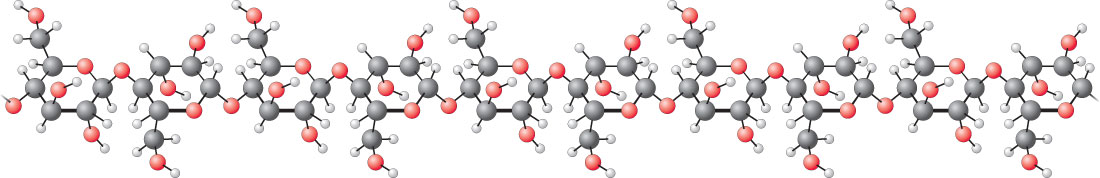
The number of protons in the nucleus defines to what chemical element the atom belongs: for example, all copper atoms contain 29 protons. The number of electrons influences the magnetic properties of an atom. Atoms can attach to one or more other atoms to form chemical compounds called molecules.