Page 21 - Curriculum Visions Dynamic Book
P. 21
Hair bleaches and detergents
Hair contains a number of colouring compounds (pigments). Hydrogen peroxide (which contains two hydrogen and two oxygen atoms) is an oxidising agent that can be used to lighten the colour of hair. A 6% solution of hydrogen peroxide is used for this purpose. The hydrogen peroxide reacts with the pigments in the hair, oxidising them to a colourless form.
Many detergents contain oxidising agents whose purpose is to turn coloured materials into colourless ones. In this way detergents are “stain” removers.
oxidation/reduction: a reaction in which oxygen is gained/lost. (Also... More generally oxidation or reduction involves the loss or gain of electrons.)
pigment: any solid material used to give a liquid a colour.
polymerisation: a chemical reaction in which large numbers of similar molecules arrange themselves into large molecules, usually
long chains. This process usually happens when there is a suitable catalyst present. For example, ethene reacts to form polythene in the presence of certain catalysts.
resin: natural or synthetic polymers that can be moulded into solid objects or spun into thread.
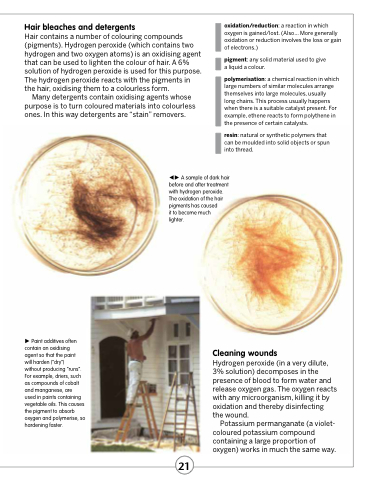
A sample of dark hair before and after treatment with hydrogen peroxide. The oxidation of the hair pigments has caused
Paint additives often contain an oxidising
agent so that the paint
will harden (“dry”)
without producing “runs”. For example, driers, such as compounds of cobalt and manganese, are
used in paints containing vegetable oils. This causes the pigment to absorb oxygen and polymerise, so hardening faster.
Cleaning wounds
Hydrogen peroxide (in a very dilute, 3% solution) decomposes in the presence of blood to form water and release oxygen gas. The oxygen reacts with any microorganism, killing it by oxidation and thereby disinfecting
the wound.
Potassium permanganate (a violet-
coloured potassium compound containing a large proportion of oxygen) works in much the same way.
it to become much lighter.
21
21