Page 39 - Curriculum Visions Dynamic Book
P. 39
EQUATION: Chemical change in soda glass
Sodium carbonate + silica ➪ soda glass + carbon dioxide Na2CO3 + SiO2(s) ➪ Na2SiO3(s) + CO2(g)
EQUATION: Chemical change in lime glass
Calcium carbonate + silica ➪ lime glass + carbon dioxide CaCO3(s) + SiO2(s) ➪ CaSiO3(s) + CO2(g)
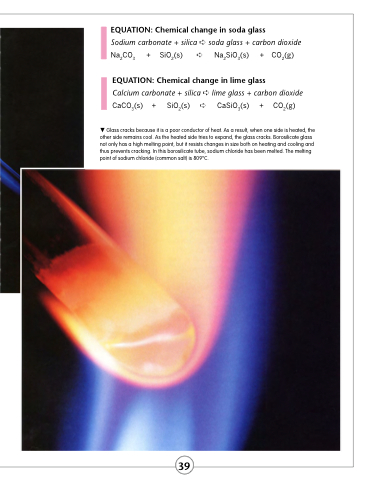
Glass cracks because it is a poor conductor of heat. As a result, when one side is heated, the other side remains cool. As the heated side tries to expand, the glass cracks. Borosilicate glass not only has a high melting point, but it resists changes in size both on heating and cooling and thus prevents cracking. In this borosilicate tube, sodium chloride has been melted. The melting point of sodium chloride (common salt) is 809°C.
39
39