Page 33 - Curriculum Visions Dynamic Book
P. 33
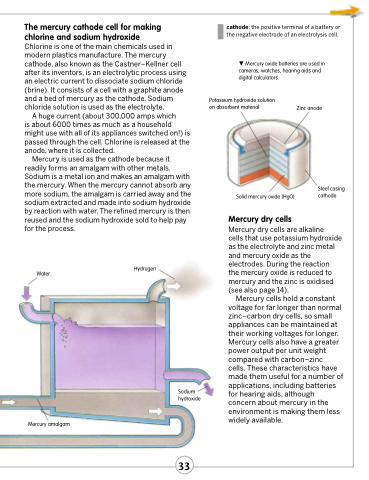
The mercury cathode cell for making chlorine and sodium hydroxide
Chlorine is one of the main chemicals used in modern plastics manufacture. The mercury cathode, also known as the Castner–Kellner cell after its inventors, is an electrolytic process using an electric current to dissociate sodium chloride (brine). It consists of a cell with a graphite anode and a bed of mercury as the cathode. Sodium chloride solution is used as the electrolyte.
A huge current (about 300,000 amps which
is about 6000 times as much as a household might use with all of its appliances switched on!) is passed through the cell. Chlorine is released at the anode, where it is collected.
Mercury is used as the cathode because it readily forms an amalgam with other metals. Sodium is a metal ion and makes an amalgam with the mercury. When the mercury cannot absorb any more sodium, the amalgam is carried away and the sodium extracted and made into sodium hydroxide by reaction with water. The refined mercury is then reused and the sodium hydroxide sold to help pay for the process.
cathode: the positive terminal of a battery or the negative electrode of an electrolysis cell.
Mercury oxide batteries are used in cameras, watches, hearing aids and digital calculators.
Potassium hydroxide solution on absorbent material
Zinc anode
Water
Hydrogen
Mercury dry cells are alkaline
cells that use potassium hydroxide as the electrolyte and zinc metal and mercury oxide as the electrodes. During the reaction the mercury oxide is reduced to mercury and the zinc is oxidised (see also page 14).
Mercury cells hold a constant voltage for far longer than normal zinc–carbon dry cells, so small appliances can be maintained at their working voltages for longer. Mercury cells also have a greater power output per unit weight compared with carbon–zinc
cells. These characteristics have made them useful for a number of applications, including batteries for hearing aids, although concern about mercury in the environment is making them less widely available.
Sodium hydroxide
Solid mercury oxide (HgO)
Mercury dry cells
Steel casing cathode
Mercury amalgam
33
33